Our research is broadly focused on the development of useful organic materials for electronics and biological applications. In particular, we have made contributions to the advancement in the chemistry of π-electron materials containing main group elements. We strongly drive our curent research along the following four directions.
- M. Hirai, N. Tanaka, M. Sakai, S. Yamaguchi, Chem. Rev., 2019, 119, 8291-8331.
- S. Yamaguchi, A. Fukazawa, M. Taki, J. Synth. Org. Chem., Jpn., 2017, 75, 1179-1187.
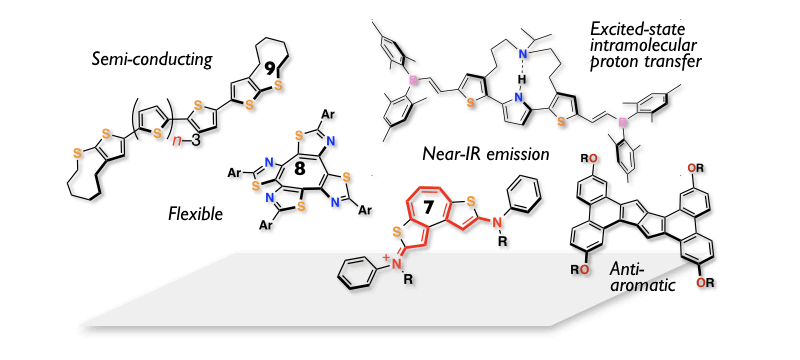
Designing New Fascinating π-Skeletons
Progress of organic (opto)electronic technologies highly relies on the development of new useful π-conjugated skeletons. We have so far produced a variety of intriguing π-conjugated systems by making best use of five-membered heteroaromatics like thiophene or seven-membered aromatic ring systems like tropyrium ion. The other approaches to this end is to utilize antiaromatic ring systems exemplified by pentalenes, or eight- and nine-membered ring skeletons. The latter designs particularly enable us to impart flexibility and thereby new functions to π-skeletons, which gives rise to fascinating properties, such as unusual fluorescence with dynamic structure changes in the excited state and high solubility for organic semiconducting materials. The state-of-the-art synthesis, including intramolecular alkyne cyclization, cross-coupling reactions, and direct C-H functionalization, is the basis of this chemistry. These "curiosity-driven" researches from a structural organic chemistry point of view would produce useful functional π-materials, and thereby contribute to the advances in organic electronics.
- K. Asai, A. Fukazawa, S. Yamaguchi, Angew. Chem. Int. Ed., 2017, 56, 6848-6852.
- H. Oshima, A. Fukazawa, S. Yamaguchi, Angew. Chem. Int. Ed., 2017, 56, 3270-3274.
- A. Fukazawa, Y. Toda, M. Hayakawa, A. Sekioka, H. Ishii, T. Okamoto, J. Takeya, Y. Hijikata, S. Yamaguchi, Chem. Eur. J., 2018, 13, 1616-1324.
- N. Suzuki, K. Suda, D. Yokogawa, H. Kitoh-Nishioka, S. Irle, A. Ando, L. M. G. Abegão, K. Kamada, A. Fukazawa, S. Yamaguchi, Chem. Sci., 2018, 9, 2666-2673.
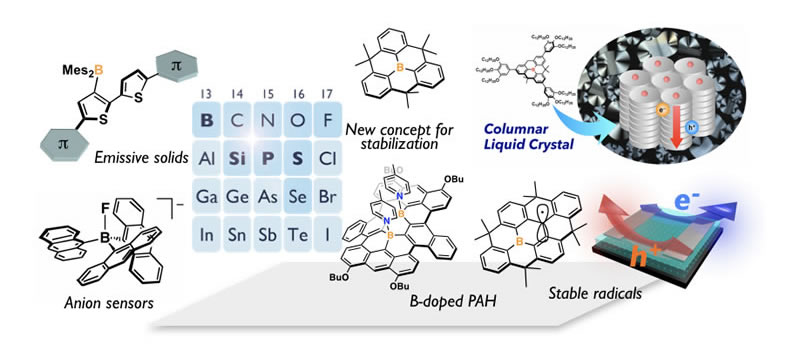
Making Best Use of Main-Group Elements
Our robust design strategy to develop new optoelectronic materials is to incorporate main group elements into π-conjugated skeletons. Among various main-group elements, B, Si, P, and S are of our particular interest. Making best use of an orbital interaction between a π-skeleton and a main-group moiety enables us to produce intriguing π-skeletons with unusual electronic structure. To gain an optimal orbital interaction, structural constraint plays a crucial role. This is also important to gain high chemical/photo-stability. Based on this strategy, we have so far synthesized various types of functional π-electron materials. Boron-embedded polycyclic aromatic hydrocarbons (PAHs) are our recent representative examples. We demonstrated that the structural constraint of triarylborane skeletons into a planar fashion sufficiently stabilizes the molecules even in the absence of steric protection. We have uncovered the intriguing properties of the boron-PAHs, derived from the empty π-orbital of the boron center, and thereby produced various promising materials including light-emitting materials for OLEDs, liquid crystal materials, and stable π-radical materials.
- Z. Zhou, A. Wakamiya, T. Kushida, and S. Yamaguchi, J. Am. Chem. Soc., 2012, 134, 4529-4532.
- T. Kushida, S. Shirai, N. Ando, T. Okamoto, H. Ishii, H. Matsui, M. Yamagishi, T. Uemura, J. Tsurumi, S. Watanabe, J. Takeya, S. Yamaguchi, J. Am. Chem. Soc. 2017, 139, 14336-14339.
- N. Ando, H. Soutome, S.Yamaguchi, Chem. Sci., 2019, 10, 7816-7821.
Pursuing Molecular Functions through Supramolecular Approach
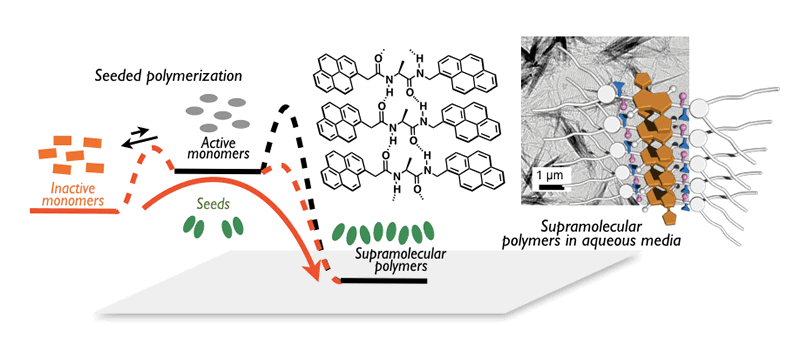
One of the most attractive features for π-materials is dramatic changes in properties upon formation of aggregates. In other words, precise control over the aggregate structure of π-materials enables us to produce unusual properties and thereby intriguing functions. From this point of view, we are working on the control of non-covalent interactions, such as π-stacking and CH---π interaction, to produce J-type aggregates, two-dimensional sheets, and nanoparticles. Our particular interest is to achieve living supramolecular polymerization of π-materials. Making use of a kinetically trapped state to suppress a spontaneous assembly, we succeeded in initiating the supramolecular polymerization while controlling over length and polydispersity of aggregates. An amino-acid-based diamide is our key substructure to realize the precise supramolecular polymerization, based on which we have succeeded in producing various fascinating assemblies. We are also pursuing their physical properties and functions based on the advanced quantum chemical calculations as well as the state-of-the-art spectroscopies.
- S. Ogi, K. Matsumoto, S. Yamaguchi, Angew. Chem. Int. Ed., 2018, 57, 2339-2343.
- S. Ogi, N. Fukaya, Arifin, B. B. Skjelstad, Y. Hijikata, S. Yamaguchi, Chem. Eur. J., 2019, 25, 7303-7307.
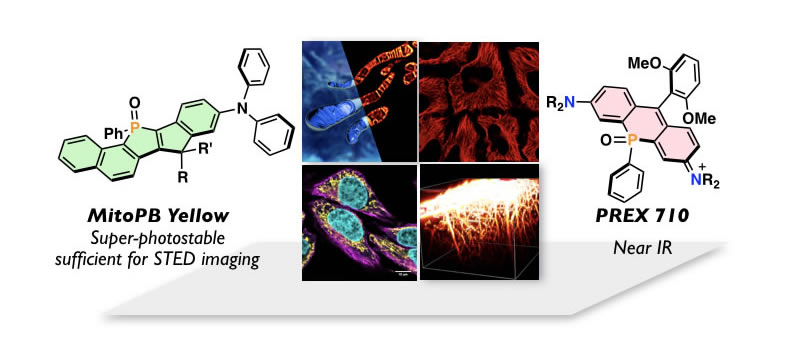
Tackling Life Science through Innovative Fluorescence Imaging
Optical imaging is one of the indispensable tools in the current biology. To open a new avenue in this technique relies on the development of useful fluorescence dyes. One of the most serious obstacles in the current fluorescence imaging is rapid photobleaching of dyes. This issue is more serious in super-resolution imaging, such as STED (stimulated emission depletion) microscopy. In this regard, we have developed a series of new fluorescent dyes consisted of rigid π-skeletons embedding a phosphine oxide (P=O) moiety, named as "PhoxBright". Their outstanding photostability enabled acquiring not only 3-D structures of cytoskeletons, but also mitochondrial inner-membrane dynamics in the living cells by conducting the STED imaging. Near-infrared (NIR) fluorescent dyes is the other target molecules in our research, which have several advantages, such as diminishing photo-damage to bio-samples, minimal interference from cell autofluorescence, as well as deep penetration in biological tissues. We succeeded in development of novel NIR dyes by embedding a P=O moiety to xanthene skeletons. PREX 710, a P=O-containing rhodamine, thus prepared showed outstanding practical utility in a range of applications, including single molecule imaging, multi-color imaging, and deep-tissue imaging. With these sophisticated dyes in hand, together with state-of-the-art imaging instrumentations, we are currently conducting interdisciplinary researches to tackle the advancement of the biology and thereby medical diagnosis.
- C. Wang, M. Taki, Y. Sato, A. Fukazawa, T. Higashiyama, S. Yamaguchi S, J. Am. Chem. Soc. 2017, 139, 10374-10381.
- C. Wang, M. Taki, Y. Sato, Y. Tamura, H. Yaginuma, Y. Okada, S. Yamaguchi, Proc. Natl. Acad. Sci. USA, 2019, 116, 15817-15822.
- M. Grzybowski, M. Taki, K. Senda, Y. Sato, T. Ariyoshi, Y. Okada, R. Kawakami, T. Imamura, S. Yamaguchi, Angew. Chem. Int. Ed. 2018, 57, 10137-10141.